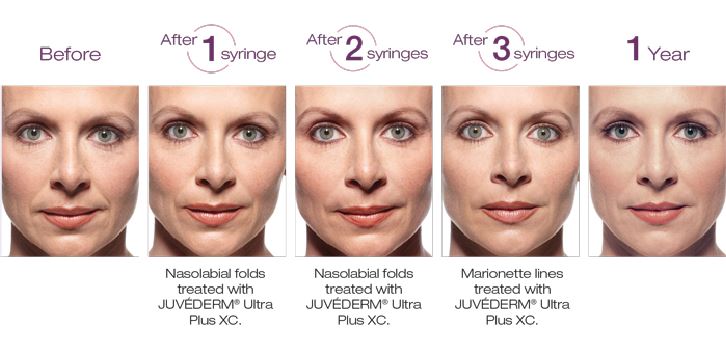
JUVÉDERM® XC is a non-surgical hyaluronic acid (HA) filler FDA-approved to instantly smooth moderate to severe wrinkles around your nose and mouth for up to one year with optimal treatment.
JUVÉDERM® injectable gel is injected into areas of facial tissue where moderate to severe facial wrinkles and folds occur to temporarily add volume to the skin, which may give the appearance of a smoother surface.
JUVÉDERM® XC Important Information
JUVÉDERM® injectable gel is injected into areas of facial tissue where moderate to severe facial wrinkles and folds occur to temporarily add volume to the skin, which may give the appearance of a smoother surface.
IMPORTANT SAFETY INFORMATION
Your physician will ask about your medical history to determine if you are an appropriate candidate for treatment. The product should not be used in patients who have:
• Severe allergies marked by a history of anaphylaxis or history or presence of multiple severe allergies
• A history of allergies to lidocaine or Gram-positive bacterial proteins
The safety and effectiveness for the treatment of areas other than facial wrinkles and folds (such as lips) have not been established in controlled clinical studies.
The following are important treatment considerations for you to discuss with your physician and understand in order to help avoid unsatisfactory results and complications:
- Patients who are using substances that can prolong bleeding, such as aspirin or ibuprofen, as with any injection, may experience increased bruising or bleeding at the injection site. You should inform your physician before treatment if you are using these types of substances
- If laser treatment, chemical peeling, or any other procedure based on active dermal response is considered after treatment with JUVÉDERM®, there is a possible risk of an inflammatory reaction at the treatment site
- JUVÉDERM® injectable gel should be used with caution in patients on immunosuppressive therapy, or therapy used to decrease the body’s immune response, as there may be an increased risk of infection
- The safety for use during pregnancy, in breastfeeding females, or in patients under 18 years has not been established
- The safety in patients with a history of excessive scarring (eg, hypertrophic scarring and keloid formations) and pigmentation disorders has not been studied
Most side effects are mild or moderate in nature, and their duration is short lasting (7 days or less).
The most common side effects include, but are not limited to, temporary injection-site reactions such as: redness, pain/tenderness, firmness, swelling, lumps/bumps, bruising, itching, and discoloration.
As with all skin-injection procedures, there is a risk of infection.
To report a problem with JUVÉDERM®, please call Allergan Product Surveillance at 1-800-624-4261.
For more information, please see the About Safety page at www.juvederm.com or call the Allergan Product Support line at 1-800-433-8871.
JUVÉDERM® injectable gel is available by prescription only.
